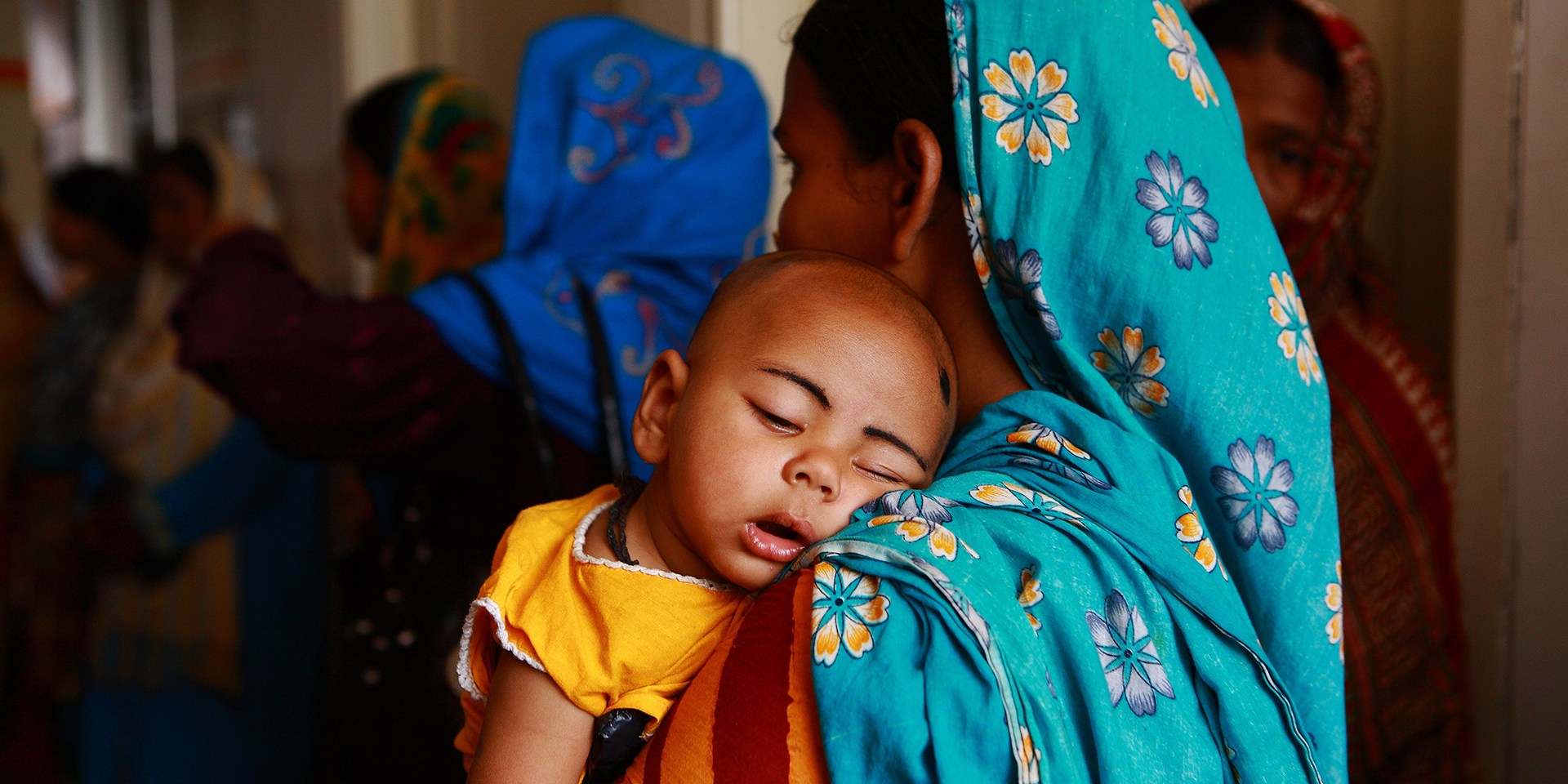
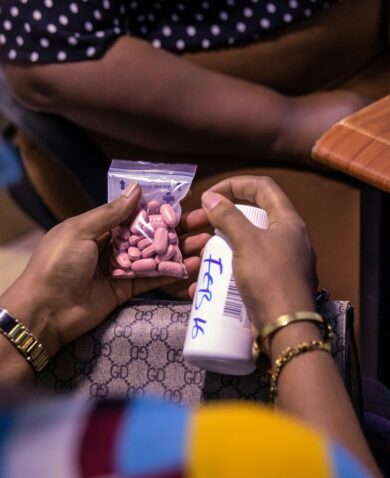
That’s the bad news. The good news is we do have the technology to prevent these deaths: a medicine that has been in use for over one hundred years — oxytocin.
Yet, one of the breakdowns in health systems that contributes to maternal deaths is that quality-assured oxytocin is often unavailable when and where women need it. Oxytocin is a heat-sensitive product that requires transportation and storage in the cold chain. Storage at room temperature or higher can result in product degradation, especially in areas with tropical climates. While the risks associated with exposure to room temperatures or higher are well known, oxytocin is still not appropriately stored during distribution, in warehouses, and at health centers and hospitals.
In an effort to tackle this issue and increase availability of quality oxytocin, the Global Health Supply Chain Program — Procurement and Supply Management (GHSC-PSM) project, in collaboration with the Maternal Health Supplies Caucus of the Reproductive Health Supplies Coalition and PATH, brought together a diverse coalition of medicine manufacturers, national governments, universities, non-governmental organizations, and global health practitioners in October of last year to review current evidence around oxytocin and come up with evidence-based, actionable recommendations. Following two days of intense review of state-of-the-art technical information on the current state of oxytocin management, the key recommendations that the participants agreed on can be summarized as “buy good stuff and keep it cold!” In more formal terms:
- Procure only oxytocin which is quality-assured and labeled for storage between 2 and 8 degrees C, as inconsistent labelling has led to confusion around storage temperature requirements for oxytocin.
- Always store oxytocin between 2 and 8 degrees C, even if the label indicates it can be kept at higher temperatures.
- Where possible, integrate oxytocin storage and distribution into existing cold chains, such as those for vaccines.