3 Ways to Better Protect Medicine Quality and Safety in Supply Chains
June 6, 2019 | 5 Minute ReadWouldn’t you want to know if the milk you were buying was spoiled or your vegetables were rotten on the inside? In global health supply chains, it’s not just about what you deliver or when you deliver it, but it’s also critical to consider “how” we deliver these vital commodities. Ensuring the medicines and other health commodities we provide are safe for use is just as important as availability and delivering on time.
Medicines distributed at health facilities in small towns in Mauritania, large cities in Nigeria, and remote areas in Cambodia take a long journey to get there. It all starts with the manufacturers that produce them — many of which are based as far away as China or India. Once they have been produced by the manufacturer, the medicines continue their journey through the supply chain with all stakeholders sharing a common goal: safe, high-quality products delivered to the patient.
As key players in the health supply chain in over 60 countries around the globe, we – and other actors in the supply chain – have a critical role to play to better manage product conditions and then respond to what we learn to ensure the safety and quality of health commodities.
On route to the patient, health commodities experience a variety of environments — from the manufacturing facility all the way down the chain to the health facility (or other distribution point). Along each step of the journey, temperature and relative humidity fluctuations during storage and distribution can have a negative impact on the quality and safety of pharmaceutical products, particularly when products are exposed to temperatures outside their prescribed ranges.
When a temperature-sensitive product is subject to such excursions, patients could pay the ultimate price. Failure to store medicines according to manufacturers’ guidance can increase the risk of degradation. For example, excessive bleeding after childbirth — postpartum hemorrhage — is the leading cause of maternal mortality worldwide, and oxytocin is the highly effective, temperature-sensitive drug of choice for prevention and treatment. Storing oxytocin at room temperature or higher can result in product degradation. The Reproductive Health Supplies Coalition’s Advocacy Messaging Framework highlights that universal access to oxytocin could save 1.4 million lives over the next 10 years, but if the cold chain is not maintained the drug loses its efficacy and threatens the lives of women who could be saved.
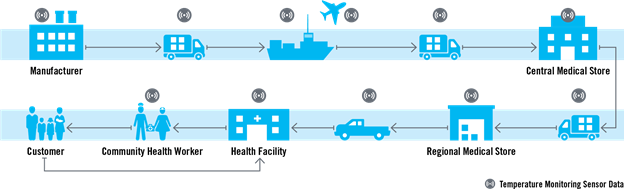
There are three vital elements and next steps needed to ensure improved quality and efficacy of health commodities moving along the supply chain: data, partnerships, and policies.
- Using a new tool to enhance data visibility about temperature fluctuations along the supply chain. Previously, data on temperature and humidity fluctuations of health commodities was only captured at the central warehouse level. However, several Chemonics projects responsible for various components of the global health supply chain, including the USAID Global Health Supply Chain Program-Procurement and Supply Management (GHSC-PSM) project, have asked ourselves: How we can better manage product temperature and the relative humidity fluctuations along the entire supply chain until the last mile to ensure the efficacy and quality of products? This led to the development of a new tool to monitor the fluctuations in the temperature during the storage and transportation of health commodities. Thanks to the internet of things (IoT), Chemonics used smart technology in the form of temperature and humidity monitoring sensors that can be installed along the supply chain to provide greater visibility into the conditions experienced by health commodities. We have already implemented this technology in Mauritania, Mozambique, Burkina Faso, and Guinea, with opportunities to scale to other countries as well.
- Forging collaboration with supply chain stakeholders. Although this tool provides accurate data to warn us when a product is compromised, there are still questions about how to best share and act on this data, how stakeholders may respond, and the extent to which this data may be compromised by conditions on the ground.The risks around medicine safety and efficacy cannot be claimed as solely the manufacturer’s responsibility; the responsibility lies with stakeholders along the rest of the supply chain. Therefore, quality and safety of health products requires strong collaboration between the manufacturers who have the stability data — meaning they know the period of time during which a multidose product can be used while retaining quality once opened — and the buyer who will transport, store, and deliver the products to the patients. Collaboration between these two groups, as well as among distributors, regulatory authorities, donors, and subject matter experts will help all stakeholders define and address the negative impact of temperature excursions on shelf life, efficacy, and safety of products. In Mauritania, we are bringing together in-country stakeholders such as the Ministry of Health and the Central Medical Store (CMS) and donors such as the Global Fund to Fight AIDS, Tuberculosis and Malaria and UNFPA to improve storage conditions in the CMS and mitigate temperature excursions. After we installed temperature monitoring sensors in several refrigerators in the CMS, we discovered they were malfunctioning and advised the relevant stakeholders to replace them. Additionally, we’re also installing sensors in refrigerated trucks that transport commodities to regional warehouses and health facilities to ensure they are working correctly. This data is regularly reported in real-time to the CMS.
- Devising and implementing standard policies and procedures. In addition to having the right data and effective partnerships with key players, supply chain stakeholders need to abide by clear policies that govern the global supply chain. Although WHO guidelines exist, there is no enforcement mechanism to ensure countries and supply chain stakeholders abide by these recommendations. Furthermore, while National Drug Regulatory Agencies (NRDA) in each country are responsible for developing their own guidelines and standard operating procedures (SOPs) for maintaining temperature excursions, these don’t often exist or still lack a means of enforcement. Supply chain actors — including governments and in-country stakeholders — need to jointly develop SOPs that tackle some of the key outstanding questions such as: When excursions occur, at what point do we need to mark and reduce shelf life of the medicine? At what point are the medicines quarantined and destroyed? And when medicines are marked to be destroyed, is this done safely in accordance with medical waste management policies and practices and in such a way as to prevent theft and misuse? SOPs are crucial for defining the correct course of action. In addition, it’s recommended that temperature monitoring be included in any quality management systems in place.
Expired or counterfeit medications, which have a higher probability of slipping into a supply chain with unregulated temperatures, are as ineffective as placebos and can sometimes have adverse, unintended impacts on patients’ health. Global supply chain actors, especially those working in countries with weak supply chains or where there is limited visibility across the supply chain, must work together to develop and implement innovative solutions that track temperature deviations. As an implementing partner, we’re doing our part to devise the necessary tools and collect the right data, but we’re appealing to the global community to work together with us and other key actors and create policies, procedures, and mechanisms for accountability to ensure that the quality and safety of health commodities is maintained throughout the supply chain.
Posts on the Chemonics blog represent the views of the authors and do not necessarily represent the views of Chemonics.